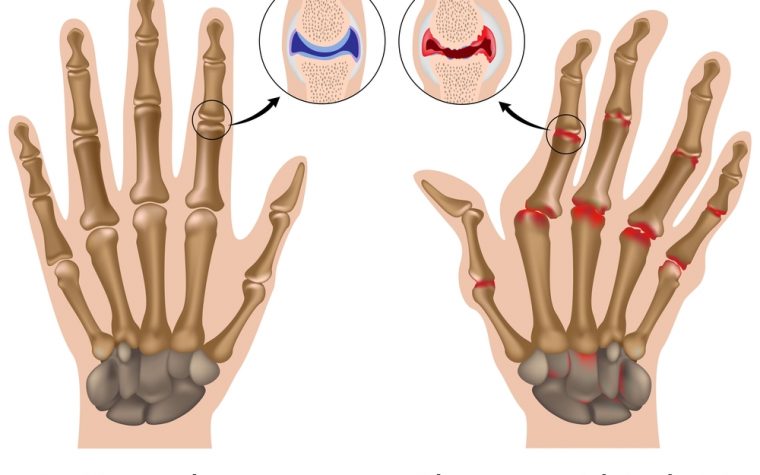
Diplomat Pharmacy Begins Dispensing Kevzara to Treat Rheumatoid Arthritis
Diplomat Pharmacy has begun filling prescriptions for Kevzara (sarilumab) for patients with moderate to severe rheumatoid arthritis (RA). The announcement comes after the U.S. Food and Drug ... Read more