 Global pharmaceutical and healthcare products company GlaxoSmithKline (GSK) has launched the “Patient Rheumatoid Arthritis Data from the Real World” (PARADE) clinical study, using Apple iPhones to monitor activity and vital signs, and collect patient feedback from rheumatoid arthritis (RA) patients.
Global pharmaceutical and healthcare products company GlaxoSmithKline (GSK) has launched the “Patient Rheumatoid Arthritis Data from the Real World” (PARADE) clinical study, using Apple iPhones to monitor activity and vital signs, and collect patient feedback from rheumatoid arthritis (RA) patients.
The iPhones of study participants are enabled to serve as mobile biosensor devices by GSK’s PARADE app, providing detailed, accurate data on subjects’ physiology and behavior, potentially enabling the company to improve its understanding of RA and patient response to medication, and to use information gathered to help with decision-making in the development of new medicines.
 The GSK PARADE app was developed using Apple’s ResearchKit and Open Source software framework designed specifically for medical research, helping doctors and scientists gather patient data more frequently and accurately. In March 2016, Apple upgraded and expanded ResearchKit with the launch of CareKit, a complimentary software framework enabling developers to build apps that empower users to take a more active role in managing their own medical conditions.
The GSK PARADE app was developed using Apple’s ResearchKit and Open Source software framework designed specifically for medical research, helping doctors and scientists gather patient data more frequently and accurately. In March 2016, Apple upgraded and expanded ResearchKit with the launch of CareKit, a complimentary software framework enabling developers to build apps that empower users to take a more active role in managing their own medical conditions.
iPhone apps built using CareKit make it easier for users to keep track of care plan schedules and to monitor symptoms and medication, and to share information with doctors, care teams, or family members about their health and any change in condition.
GSK is seeking volunteers to monitor their health and share insights of how rheumatoid arthritis affects their lives. Those who are 21 or older and diagnosed with RA may be able to participate in the study. This particular study will not provide diagnosis or treatment, but participants will be able to view their own study data and learn more about their condition. For more information or to download the free PARADE app, visit: https://itunes.apple.com/app/id1116992030
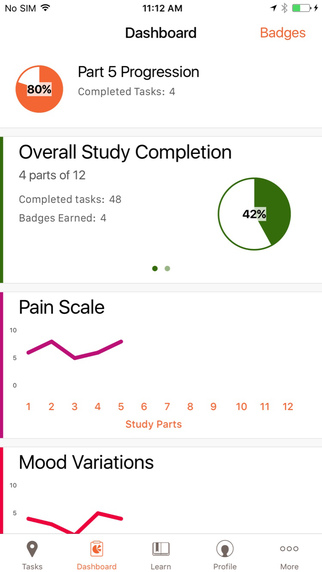 According to GSK, its rheumatoid arthritis study using the GSK PARADE iPhone app marks the first time ResearchKit has been used to support clinical research, in this instance examining the impact RA has on daily life. The study will conduct surveys and use the iPhone’s sensors to collect and track common rheumatoid arthritis symptoms: joint pain, fatigue, mood alteration, walk, and quality of life, tracking activity and quality of life data for 300 patients over a three-month period.
According to GSK, its rheumatoid arthritis study using the GSK PARADE iPhone app marks the first time ResearchKit has been used to support clinical research, in this instance examining the impact RA has on daily life. The study will conduct surveys and use the iPhone’s sensors to collect and track common rheumatoid arthritis symptoms: joint pain, fatigue, mood alteration, walk, and quality of life, tracking activity and quality of life data for 300 patients over a three-month period.
Data gathered will help GSK design studies to help them develop medicines more effectively, explaining that a medicine’s “development process starts with learning from real patients by including their insights and health goals into our research.”
Through the CareKit’s Care Card module, users can track actions like taking medication or completing physical exercises, with their activities automatically calculated and entered using sensors such as the iPhone’s accelerometer and gyroscope. CareKit’s Symptom and Measurement Tracker lets users record their symptoms and convey how they’re feeling in real time — for example, monitoring body temperature for possible infections, or measuring pain or fatigue.
Progress updates can include simple surveys, photos that capture the progression of a wound, or activities calculated by using iPhones, like quantifying range of motion. The Insight Dashboard module contains the user’s personal data, which they can share with healthcare providers in conversations regarding treatment plans, and maps symptoms against action items in Care Card to show how treatments are working.
 “With ResearchKit, we quickly realized the power of mobile apps for running inexpensive, high-quality clinical studies with unprecedented reach,” said Ray Dorsey, MD, the David M. Levy Professor of Neurology at the University of Rochester Medical Center in an Apple press release. “It’s opening up a whole new opportunity for the democratization of research and medicine.”
“With ResearchKit, we quickly realized the power of mobile apps for running inexpensive, high-quality clinical studies with unprecedented reach,” said Ray Dorsey, MD, the David M. Levy Professor of Neurology at the University of Rochester Medical Center in an Apple press release. “It’s opening up a whole new opportunity for the democratization of research and medicine.”
GSK notes that the ability to collect patient data from mobile devices may, in the future, also help reduce the burden of patients participating in clinical studies by reducing the frequency of patients having to travel to their doctors’ offices for monitoring, potentially disrupting the traditional research model.


